What are teeth made of? I was asked by my baby, who had just finished brushing his teeth. I’m sure many parents have experienced a similar scenario. When a curious child brushes his or her teeth, the question inevitably arises out of nowhere. So, let’s explore the composition, structure, and chemical makeup of teeth through this article.
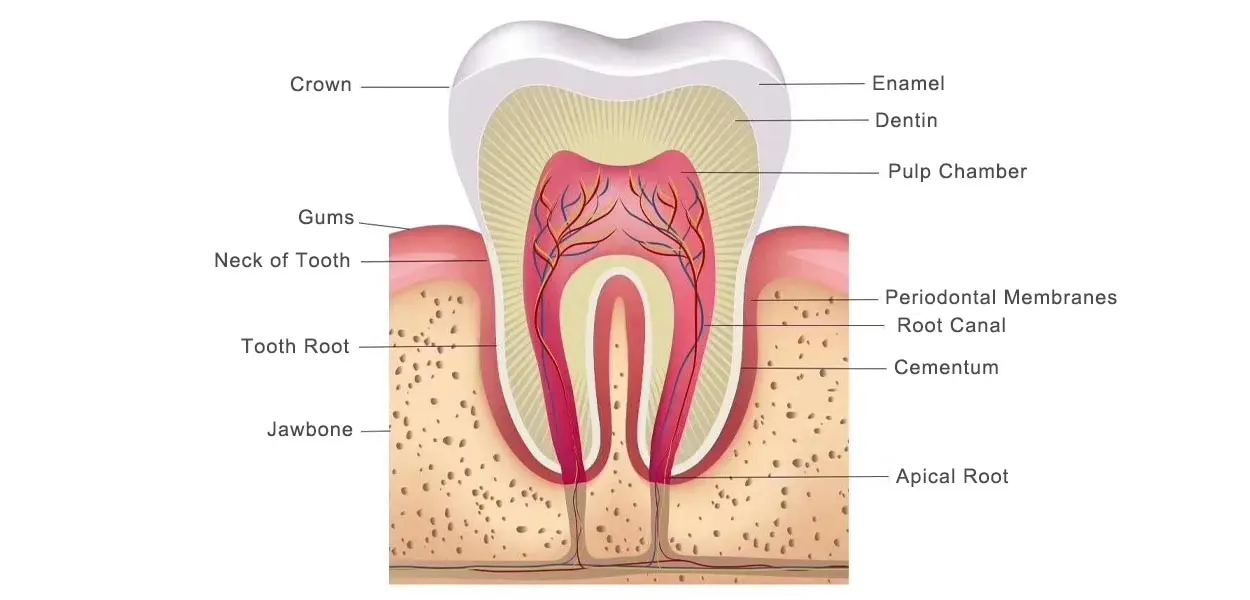
1. The basic structure of teeth
Teeth are made of highly mineralized biological material with a multilayered structure that provides a perfect balance of hardness and toughness. It consists of four main types of mineralized tissues:
Enamel:
Enamel is the outermost layer of the tooth and the hardest tissue in humans, covering the surface of the crown. It is translucent and milky white, with crystals arranged in a honeycomb pattern. Its main function is to protect the tooth from external damage.
- Composition: 95-97% inorganic, mainly hydroxyapatite crystals (calcium phosphate mineral Ca10(PO4)6(OH)2), with traces of fluoride, magnesium and carbonate ions.
- Disadvantages: no cellular structure, no regeneration after physical abrasion or acid erosion (caries), but fluoride can enhance the ability of acid resistance.
Dentin:
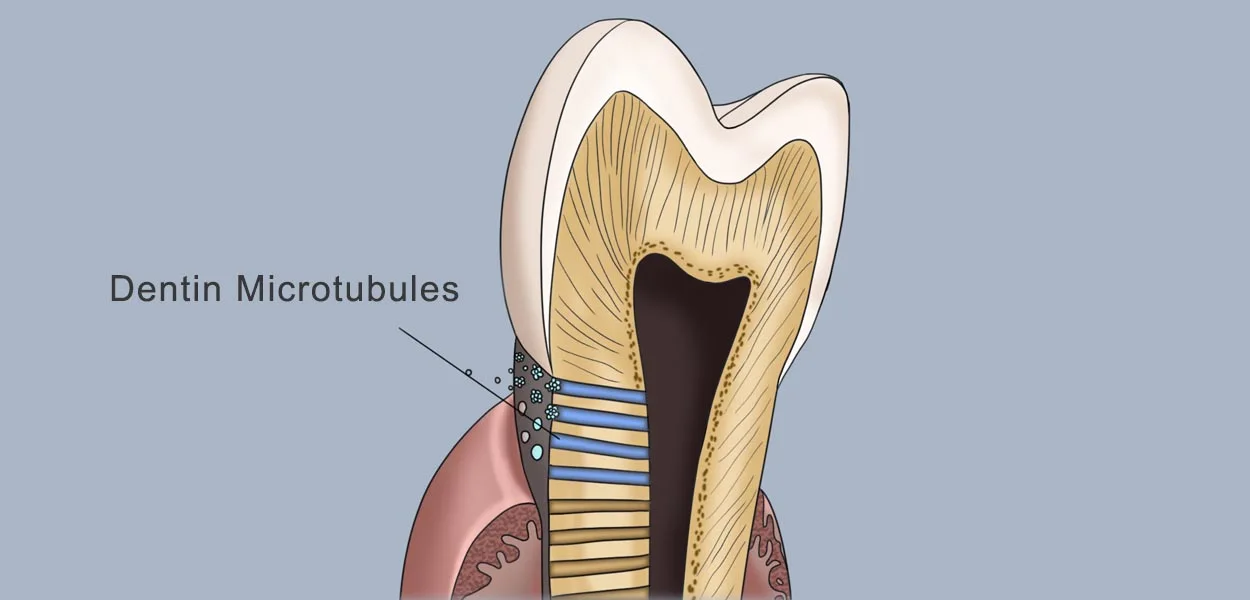
Below the enamel, dentin makes up most of the tooth volume. It is slightly softer than enamel and contains 30,000 microtubules per mm², which form a hydraulic sensing system. These microtubules transmit masticatory pressure, and external stimuli trigger the movement of fluid in the microtubules, triggering neuralgia.
- Ingredients: 70% Hydroxyapatite + 20% Collagen + 10% Moisture
- Sensitization mechanism: Enamel breakage exposes the microtubules and hot and cold stimuli reach the nerves and trigger pain. This is the reason why we often have toothaches, such as cavities that cause holes in the enamel, which can lead to tooth sensitivity and pain.
Cementum:
Covers the surface of the tooth root and is mechanically locked to the periodontal ligament by means of Sharpey’s fibers, helping to anchor the tooth firmly in the alveolar bone.
- Composition: 65% inorganic (hydroxyapatite) + 23% collagen + 12% water.
- Characteristics: Dental osteoblasts are active for life and have a high regenerative capacity to repair root surface damage.
Pulp:
The pulp is located in the deepest of the tooth and consists of blood vessels, nerves and connective tissue, and is responsible for the supply of nutrients and the transmission of pain. It also ages with age, with the pulp cavity shrinking by 3% per year and nerve density decreasing by 2% after the age of 30.
2. Clinical significance of tooth chemistry
Understanding the chemical composition of teeth is not only useful for theoretical exploration, but also has important applications in clinical practice:
Chemical nature of caries
Hydroxyapatite begins to dissolve when oral bacteria metabolize sugar to produce acids, causing the local pH to drop below the critical pH (approximately 5.5) (the enamel demineralization that occurs in the early stages of caries). The chemical principle is: Ca₁₀(PO₄)₆(OH)₂ + 8H⁺ → 10Ca²⁺ + 6HPO₄²- + 2H₂O .
Dentin due to its higher organic content, carbonate doping and dentin tubule structure, the speed of demineralization is much faster than enamel, caries once penetrated enamel tend to expand rapidly.
Fluoride can prevent caries is the principle is through the formation of fluorapatite, raising the threshold of demineralization and promote remineralization. In this way to achieve the purpose of strong caries prevention.
The cornerstone of bionic restorative materials
- Ceramic restorations (all-ceramic crowns, inlays): the pursuit of aesthetics and wear resistance close to tooth enamel.
- Composite resin: mimics the organic-inorganic composite structure of dentin.
- Glass ionized cementum: release of fluoride ions, capable of ion exchange with tooth minerals.
- Bioactive materials: development of new filling materials that induce hydroxyapatite deposition and promote dentin regeneration.
Explore the mechanism of tooth sensitivity
Exposure of dentin, e.g. after enamel wear, gum recession, external stimuli (cold, hot, sour, sweet, mechanical friction) cause rapid flow of fluid in the dentin tubules, which stimulates the nerve endings in the tubules and triggers pain. By targeting this mechanism, a treatment program can be developed to solve the problem of tooth sensitivity and toothache.
- In the short term, brushing with an anti-allergic toothpaste can temporarily seal the dentin tubules, achieving the effect of anti-allergy and pain relief.
- If you want to solve the problem of tooth sensitivity and toothache completely, you need to block the external stimulation of dentin tubules. This can be done by remineralizing the enamel, tooth filling, or making a crown to cover the exposed dentin.
Understanding the cause and effect of abnormal tooth development
- Enamel hypoplasia: Usually a systemic disease of pregnancy/infancy, malnutrition (especially vitamin A, C, and D deficiencies) affects enamel matrix formation and mineralization, resulting in structural defects, discoloration, and susceptibility to caries.
- Dentin dysplasia: often associated with inherited diseases of collagen metabolism (e.g. osteogenesis imperfecta).
3. Differences and associations between milk teeth and permanent teeth
- Milk teeth: 20 teeth with thinner enamel, guiding the path of permanent teeth eruption. Early loss of milk teeth is prone to misalignment of permanent teeth.
- Permanent teeth: 32 (including wisdom teeth), divided into incisors, cuspids, premolars, and molars by function.
4. Teeth vs. Bones: differences in composition and function
Many people think of teeth as being part of bone. Although both teeth and bones are composed of hydroxyapatite, teeth are more specialized in terms of composition and structure.
| Feature | Teeth (especially Enamel) | Bone |
|---|---|---|
| Main Function | Chewing, cutting, grinding food; aesthetics; speech | Supports body; protects organs; hematopoiesis; mineral reservoir |
| Hardness | Extremely high (enamel is the hardest tissue in the human body) | High, but lower than enamel |
| Toughness | Enamel is brittle; dentin/bone are tough | Good toughness (resists fracture) |
| Inorganic Content | Very high (enamel ~96%, dentin ~70%, bone ~65%) | ~65% (variable) |
| Organic Matrix | Very little in enamel (<1%); dentin/bone mainly type I collagen (20–23%) | Mainly type I collagen (~25%) |
| Cells and Blood Vessels | Enamel: no cells or vessels; dentin: cell processes but no bodies; pulp has cells, vessels, nerves | Rich in cells (osteocytes, osteoblasts/clasts); highly vascularized |
| Self-repair Ability | Very limited (none in enamel; dentin has limited secondary dentin formation) | Strong (continuous bone remodeling) |
| Developmental Origin | Ectoderm (enamel) + mesenchyme (other parts) | Mesenchyme |
5. Conclusion
Teeth play an important role in the human body as they are responsible for chewing, articulation and facial aesthetics. What are teeth made of? They are a combination of a fortress of hydroxyapatite crystals, a shield of collagen networks, a mineralization process regulated by sophisticated proteins, and a core of nourishing vital fluids. Understand what are teeth made of chemically and chemical composition of teeth. Discover the fundamental mechanisms that can effectively prevent and treat dental diseases, and develop better oral care products and revolutionary dental materials.